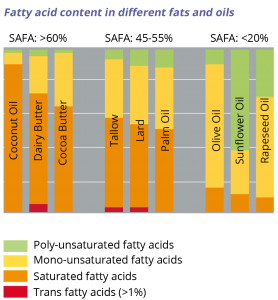
On average, palm oil has almost equal amounts of saturated and unsaturated fatty acids. All oils and fats, irrespective of their origin, contain both saturated and unsaturated fatty acids. The ratio depends on the type of oil or fat. The proportion of saturated fatty acids in palm oil compares favourably to the saturated fatty acids content of other fats of similar application, such as coconut oil, butter and cocoa butter. Because of its plant origin, variability in fatty acid composition may occur due to geographical factors, for example soil, weather and the type of oil palm tree.
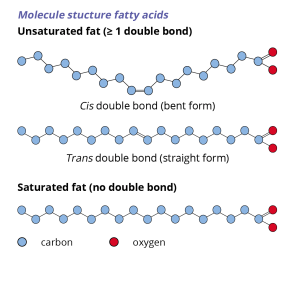
The term saturated fatty acid is often written in shorthand as SAFA. In a saturated fatty acid the carbon atoms are connected with only single bonds, which enables the fatty acids to pack closely together. Oils and fats which are rich in saturated fatty acids will have a higher melting point and a denser structure and thus will be more solid at room temperature.
Unsaturated fatty acids can be either mono-unsaturated (MUFA) or poly-unsaturated (PUFA). Unsaturated fatty acids contain one or more double bonds in their hydrocarbon chain. The double bond introduces a kink in the hydrocarbon chain, which makes it more difficult for the fatty acids to pack tightly. Oils which are rich in mono- or poly-unsaturated fatty acids are therefore often liquid at room temperature, like cooking oils.
Trans fatty acids (or TFA) are unsaturated fatty acids in which the carbon chain extends from opposite sides of the double bond. This results in a straight molecular structure with similar functional properties as SAFA.
Having a unique and balanced composition of saturated and unsaturated fatty acids, palm oil generally does not require partial hydrogenation (‘hardening’) in applications where solid fat is desirable, thus avoiding the formation of trans fatty acids.